The Vitamin That Helps–and Hurts–Cancer
[post_date]

Madeline Childress joined the NRI in 2019 and began her docotral studies in the fall of 2020. She completed her undergraduate program at the University of Alabama, where she graduated with a major in Biology and minor in Chemistry and Spanish. She is a graduate student in the Natalia Krupenko lab researching the role of folate in promoting human health and preventing disease.
“Cancer, believe it or not, is actually quite easy to kill,” said Childress. “The real challenge is not killing everything else in the process.”
Unlike viruses or bacteria, cancer cells are not foreign invaders—they’re our own cells that have gone rogue. That makes them notoriously difficult to target. But Childress is exploring a promising new mechanism in the world of anti-metabolites. Anti-metabolites are a type of cancer treatment that works by starving cancer cells of the nutrients they need to grow.
One such nutrient is folate, also known as vitamin B9. “Folate is essential for healthy cell function and even cancer prevention,” she explained. “But if cancer is already present, folate can fuel its growth. It’s a double-edged sword.”
“But if cancer is already present, folate can fuel its growth. It’s a double-edged sword.”
Folate plays a central role in a critical cellular process called one-carbon metabolism, which Childress likens to an assembly line. “It’s how cells move single carbon atoms around to build DNA and other important molecules. Disrupt that line, and the cell can’t grow or divide—which is great for stopping cancer,” she said.
Drugs known as anti-folates work by doing just that: disrupting the folate pathway. But many cancer cells adapt and survive, particularly those with mutations in a protein called p53. Known as the “guardian of the genome,” p53 normally acts as an emergency brake to stop damaged cells from multiplying. In more than half of all cancers, this brake is broken.
This “guardian of the genome” is the key to the new treatment mechanism that is being studied at the NRI. Childress explains, “p53 mutations are a huge problem in cancer biology. They’ve long been considered ‘undruggable.’ My research is focused on finding new ways to restore p53 function—or at least give cancer cells a reason to hit the brakes again.”
In her work, Childress has uncovered a fascinating twist: when cells are stressed from folate deprivation or anti-folate drugs, they produce a special fat molecule. That molecule can bind to p53, stabilize it, and help restore its tumor-fighting powers—even when mutated.
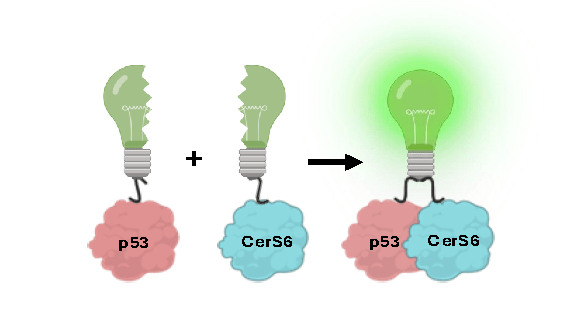
To study this interaction, she uses a technique called Bimolecular Fluorescence Complementation. “It’s kind of like splitting a glowing lightbulb in half and attaching each piece to different proteins,” she explained. “When p53 and the enzyme that makes this lipid come together, the lightbulb switches on. We can visibly see the interaction light up in the cell.”
Childress hopes her findings will lead to new approaches in cancer therapy—ones that are more targeted, less toxic, and capable of reawakening the body’s own tumor-fighting machinery. “At the end of the day, I want to help make cancer treatments smarter,” she said. “Restoring p53’s power could be a game-changer.”
Dig Deeper
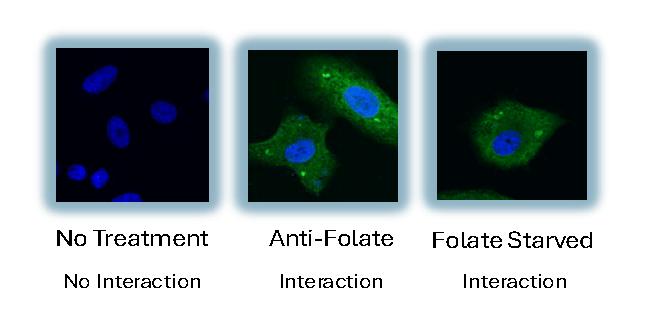
💡 This glowing effect helps scientists visually confirm when two specific proteins interact inside a living cell—bringing abstract molecular activity to light.
Figure A: Depiction of the Bimolecular Fluorescence Complementation mechanism used to visualize the interaction between p53 and the lipid molecule, CerS6.
Figure B: Visualization of real fluorescent interactions under different treatment conditions.
Images provided by UNC NRI N. Krupenko Lab.
Share on Socials
Inspired
By What You Read?
NRI scientists are discovering how genes, environment, and microbiome affect our individual requirements for nutrients so that, soon, medical practitioners will be able to guide people in their health from childhood through old age without adding to these tragic numbers. Our critical research depends on the generosity of people like you.