Beyond Direct Damage: How Alcohol Alters the Entire Pregnancy Environment
[post_date]

Dr. Smith’s laboratory studies the molecular mechanisms by which dietary components affect prenatal development. Current work largely focuses upon alcohol and how it causes Fetal Alcohol Spectrum Disorders (FASD). Smith is interested in how alcohol damages the embryo and fetus, and in the environmental and genetic factors that attenuate or heighten alcohol’s toxicity.
Prenatal alcohol exposure (PAE) is the leading known cause of preventable neurodevelopmental disability. In the United States, up to 5% of first graders meet the diagnostic criteria for fetal alcohol spectrum disorders (FASD), which are linked to learning difficulties, behavioral challenges, growth problems, and long-term health issues.1 Despite public health campaigns, rates of alcohol use during pregnancy remain stubbornly high—about 14% of pregnant women report drinking, and 4% report binge drinking.2 While scientists have long understood that alcohol can directly damage the developing brain, new research shows that its harmful reach extends far beyond the fetus itself.
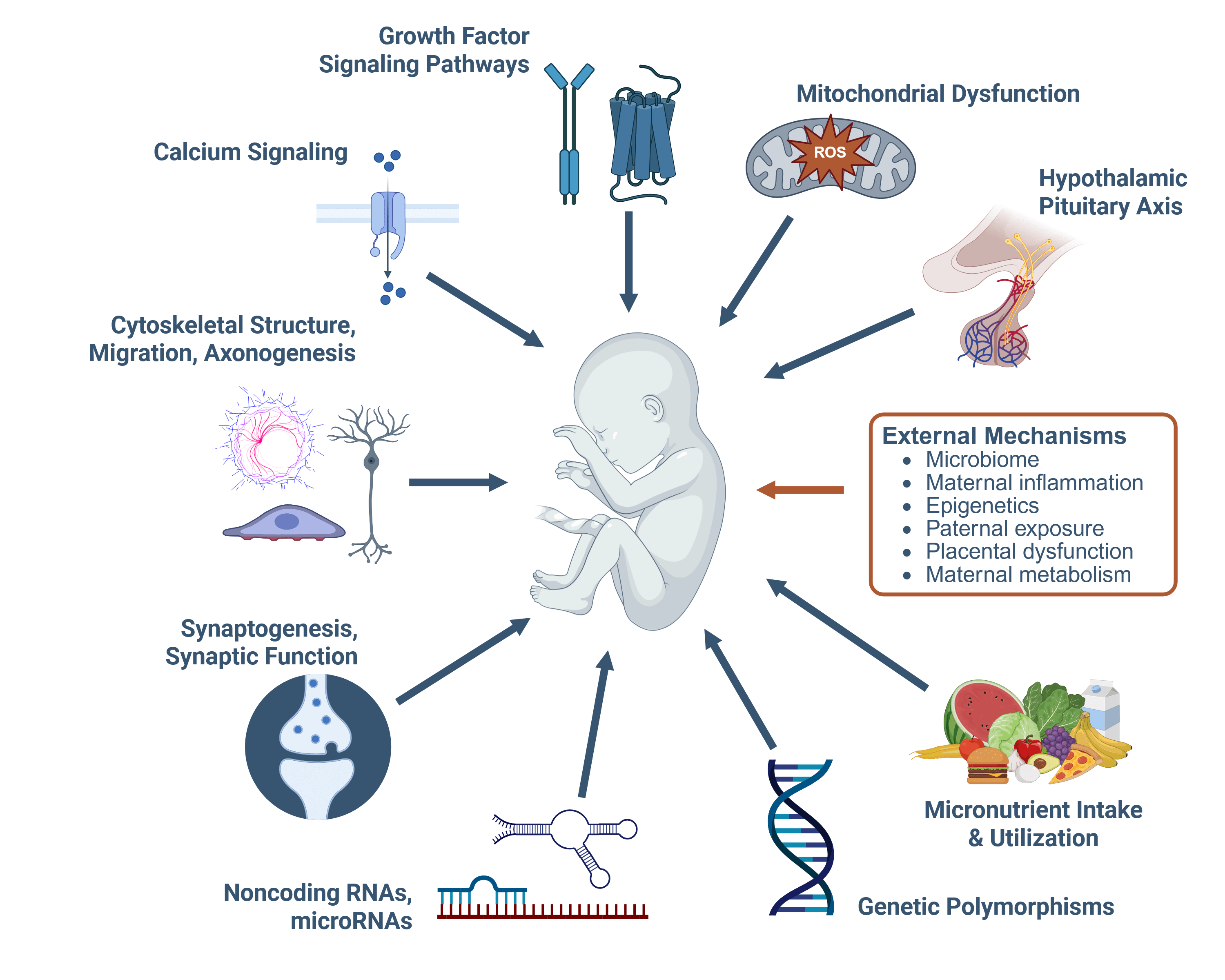
In a recent review, Susan Smith, PhD, Professor of Nutrition at the UNC Nutrition Research Institute examined “exogenous mechanisms,” processes outside of the fetus that still have a profound influence on its development.3 The findings reveal that alcohol doesn’t just act directly on the brain and body of the developing baby; it also alters the mother’s microbiome, disrupts the nutrient supply, triggers chronic inflammation, and even changes the placenta’s ability to support healthy growth. These disruptions are interconnected: for example, alcohol-related changes in gut bacteria can release toxins into the mother’s bloodstream, fueling inflammation that impairs the placenta and limits nutrient and oxygen delivery to the fetus.
One surprising insight is that the biological father’s drinking before conception may also play a role. Heavy paternal alcohol use can alter the chemical signals (epigenetic marks) carried by sperm—changes that can affect both fetal and placental development. Another key finding, identified by Nipun Saini, PhD, an assistant professor at the NRI, is that PAE can prevent pregnant women from developing the mild insulin resistance that normally helps direct more glucose to the baby. Without this adaptation, fetal growth can be restricted. Alcohol-related inflammation may also cause “functional iron deficiency” in the fetus—iron is present but locked away in storage—leading to anemia and potential brain development problems.
Dr. Smith emphasizes that these different pathways don’t act in isolation. “No mechanism is an island,” she explains. “Alcohol’s effects on the microbiome, metabolism, the placenta, and even the father’s contribution are mechanistically interwoven. Understanding how these processes interact will help us design interventions that address the root causes, not just the symptoms, of alcohol’s harm in pregnancy.”
“No mechanism is an island…understanding how these processes interact will help us design interventions that address the root causes, not just the symptoms.”
These findings open the door to new strategies for prevention and treatment. Supporting a healthy microbiome, reducing inflammation, improving nutrient delivery, and protecting placental function could all help reduce harm from prenatal alcohol exposure. Even modest improvements in one area—such as ensuring adequate iron availability—might have compounding benefits when paired with other targeted interventions.
The research also highlights gaps in our understanding. Many of these findings come from animal models which often lack diet information, even though diet has a major influence on outcomes involving the microbiome, inflammation, metabolism and more. Moreover, these mechanisms may interact differently depending on timing, dose, and individual biology. By expanding studies to include the roles of both parents, and by looking at how nutrition and environment shape these pathways, scientists can better pinpoint where interventions will make the most difference.
Alcohol exposure during pregnancy is not just a matter of direct fetal harm—it disrupts a complex, interdependent system that supports growth and development. Recognizing and addressing these broader influences could transform how we prevent and treat the effects of prenatal alcohol exposure, ultimately giving more children the chance to start life with a healthier foundation.
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, et al. Prevalence of fetal alcohol spectrum disorders in 4 US communities. Jama (2018) 319(5):474–82. 10.1001/jama.2017.21896
- Gosdin LK, Deputy NP, Kim SY, Dang EP, Denny CH. Alcohol consumption and binge drinking during pregnancy among adults aged 18-49 Years – United States, 2018-2020. MMWR Morb Mortal Wkly Rep (2022) 71(1):10–3. 10.15585/mmwr.mm7101a2
- Smith, SM. “Nonconceptus Mechanisms of Prenatal Alcohol Exposure That Disrupt Embryo-Fetal Development: An Integrative View.” Alcohol Res. 2025 45:07. DOI:10.35946/arcr.v45.1.07; PMCID:PMC12278911.
Dig Deeper
This figure, adapted from the publication referenced in this article, outlines a range of mechanisms through which alcohol exposure can affect fetal development.
💡 Key Concepts from this Figure:
Epigenetics – Refers to chemical changes that influence how genes are turned on or off without altering the DNA sequence itself. During pregnancy, epigenetic patterns help control the timing and intensity of gene activity that guides fetal growth. Disruptions—such as those caused by alcohol—can change these patterns, affecting brain development, organ function, and long-term health.
Paternal Exposure – The health and environmental exposures of the father before conception can influence fetal development through changes in sperm. For example, heavy alcohol use can alter epigenetic marks in sperm, potentially affecting both the placenta and the baby’s growth. This means both parents’ health matters before pregnancy begins.
Microbiome – The diverse community of microbes in the gut that supports digestion, immune function, and nutrient absorption. In pregnancy, the mother’s microbiome helps regulate inflammation and ensures a steady supply of nutrients to the fetus. Alcohol-related changes in the microbiome can release harmful substances into the bloodstream, impairing placental function and fetal development.
Images provided by UNC NRI Smith Lab
Share on Socials
Inspired
By What You Read?
NRI scientists are discovering how genes, environment, and microbiome affect our individual requirements for nutrients so that, soon, medical practitioners will be able to guide people in their health from childhood through old age without adding to these tragic numbers. Our critical research depends on the generosity of people like you.