This article was published originally by the Gillings School of Global Public Health on January 25, 2022.
New findings from researchers at the UNC Nutrition Research Institute (NRI) have revealed the structure of an enzyme that plays a key role in predicting whether a person might be susceptible to metabolic diseases or cancer.
ALDH1L1, a common metabolic protein found in the liver and other tissues, helps the human body regulate folate, or vitamin B9. Lack of folate can contribute to neural tube defects in newborns and has been linked to several types of cancer and cardiovascular disease. ALDH1L1 is also considered a tumor suppressor because it is consistently downregulated in malignant tumors and cancer cells.
While the effects of ALDH1L1 have been long understood, its complex structure has made it difficult for researchers to understand how it works, how mutations might alter its function and how to design drugs that target its function.

DR. SERGEY KRUPENKO
Results from a new study published in Communications Biology can now answer these questions definitively and give nutrition experts a better understanding of how to create interventions that support the critical work of this enzyme.

DR. NATALIA KRUPENKO
The study was performed at NRI in the lab of Sergey Krupenko, PhD, professor in the Department of Nutrition at the UNC Gillings School of Global Public Health, and featured work from postdoctoral researcher Valentin Sereda, PhD, and Natalia Krupenko, PhD, associate professor in the Department of Nutrition. The study was done in collaboration with Yaroslav Tsybovsky, PhD, at the National Laboratory for Cancer Research at the National Cancer Institute and Marcin Golczak, PhD, from the Case Western Reserve University School of Medicine.
“Our structure revealed a complex organization of ALDH1L1 with some unique features not common in nature,” said Sergey Krupenko. “The structure was impossible to solve by more standard approaches like x-ray crystallography or nuclear magnetic resonance. Only the application of a more advanced technique, cryo-electron microscopy, led us to the result.”
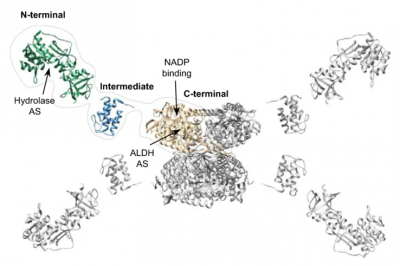
THIS IS THE STRUCTURE OF THE ALDH1L1 PROTEIN.
ALDH1L1 is a three-domain protein in which the C-terminal domain forms a tetramer and serves as the enzyme’s catalytic core. The N-terminal domain binds the substrate folate. The linker domain links N- and C-terminal domains and transfers the formyl group of the substrate between folate-binding and catalytic centers.
Through use of cryo-electron microscopy, the study team uncovered the unusual architecture of ALDH1L1, which has a tetrameric organization that enables enzyme catalysis to span multiple protein subunits (protomers). Each formyl-transferring linker domain reaches the catalytic center in a different protomer. This makes the tetrameric state of ALDH1L1 indispensable for enzyme functionality, which also involves transient domain interactions and large-scale domain movements.
“ALDH1L1 is an example when gene fusion, per se, does not produce novel functionality without higher hierarchy of structural organization. This is highly unique for a protein,” said Sergey Krupenko. “The mode of interactions between protein modules demonstrated by our study was highly unexpected, unpredictable and is not commonly seen in nature. These interactions explain why ALDH1L1 must exist as a tetramer for its functionality.
“Taking into consideration the role of ALDH1L1 as cellular proliferation regulator and putative tumor suppressor, our structure also creates an opportunity to target this protein for disease treatment.”
Because ALDH1L1 has numerous naturally occurring mutations found in different populations, the results of this study will allow experts to predict how a specific mutation might affect the enzyme’s structure and functionality. These mutations might serve as markers for the likelihood of certain metabolic diseases, including cancer. Supplementing diet with more folate is one intervention that nutrition experts can use to target specific variants.
NRI professors Sergey Krupenko and Susan Sumner, PhD, and Associate Professor Saroja Voruganti, PhD, were recently awarded a five-year grant from the National Institutes of Health to investigate the link between ALDH1L1 variants, dietary folate and metabolic health.
Contact the UNC Gillings School of Global Public Health communications team at sphcomm@unc.edu.
Posted January 27, 2022

