Sandra M. Mooney, PhD
Associate Professor of Nutrition
sandra_mooney@unc.edu
704-250-5022
View CV
Sandra Mooney, PhD joined the UNC Chapel Hill Nutrition Research Institute in August 2018 as an Associate Professor of Nutrition. Her research program investigates the effect(s) of environment and genes on brain development, with a focus on prenatal alcohol exposure. Current studies use animal models to understand how nutritional needs change after alcohol exposure, thereby increasing the chances that modifying (or personalizing) nutrition will optimize growth and development. This work is supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Dr. Mooney received her Ph.D. from the University of Otago in New Zealand.
The overall theme of Dr. Mooney’s research is to understand normal brain development, how exposure to alcohol (and other drugs or experiences) disrupts this, what the behavioral outcomes are, and whether simple nutrition-based interventions can improve outcomes. Developmental exposure to ethanol profoundly affects development of the nervous system. Indeed, fetal alcohol exposure is described as the primary known cause of mental retardation, and recent estimates suggest that 2-5% of US children can be diagnosed with a Fetal Alcohol Spectrum Disorder.
Show More

Carolyn Munson
Research Specialist, Mooney Lab
Carolyn Munson graduated in 2015 from Rowan Cabarrus Community College with an AAS in biotechnology. She is working as a research technician in the Surzenko Lab at the NRI. Much of her work is directed by Dr. Natalia Surzenko performing immunohistochemistry work.

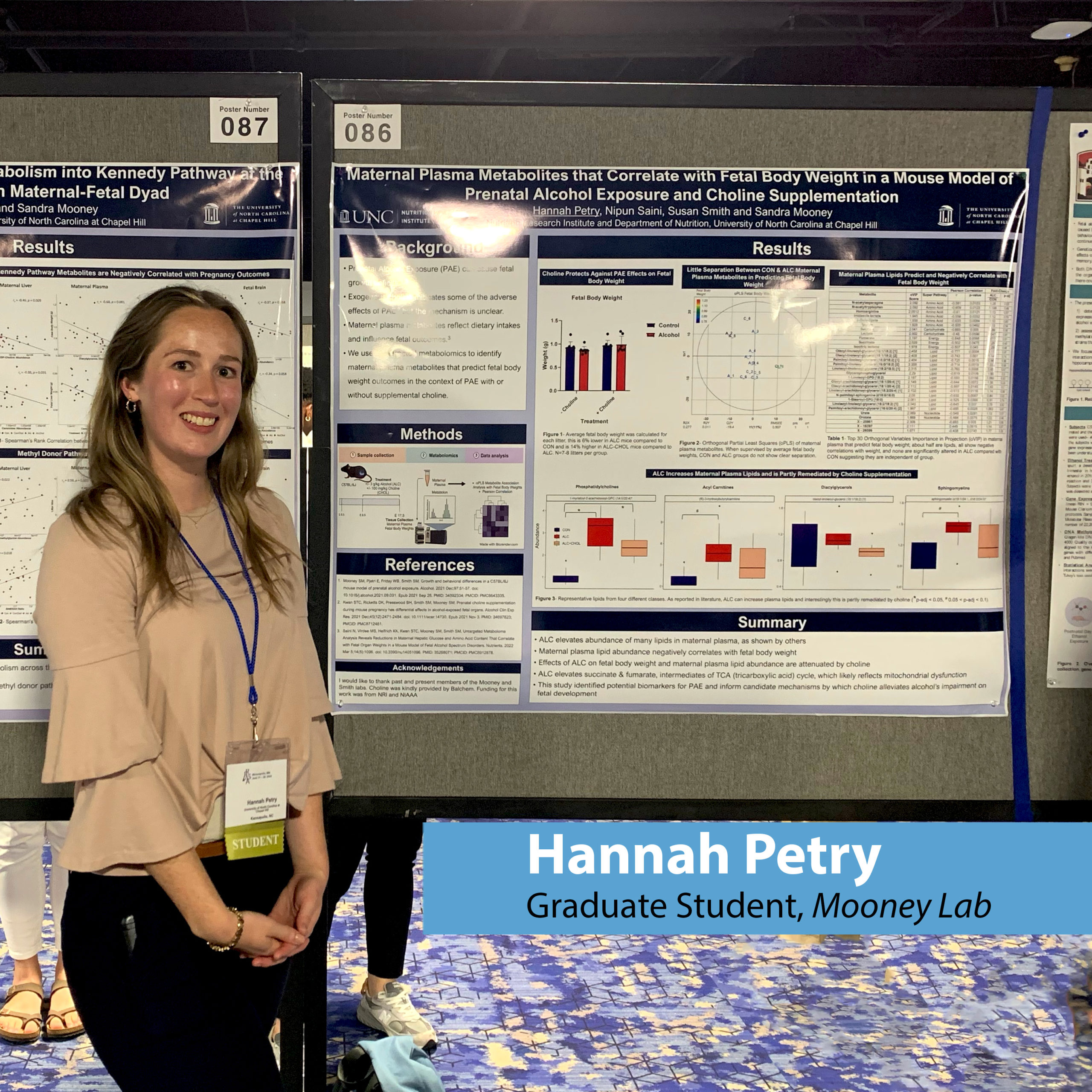
Hannah Petry
Graduate Student, Mooney Lab
Hannah obtained her Bachelor of Science in Nutritional Sciences and Dietetics in May 2022 from Texas Tech University. She joined the NRI and began her doctoral studies in Fall 2022. She works in the Mooney Lab to research fetal alcohol spectrum disorder (FASD).

Nathan Pressley
Research Technician, Mooney Lab
Nathan graduated from UNC at Greensboro in May 2023 with a BS in Psychology and a Minor in Statistics. He is from Gold Hill, NC and has family from Kannapolis. Nathan is excited to join Dr. Mooney’s lab and to learn all that he can during his time at the NRI. In his free time, he enjoys gardening, spending time with his dogs, and kayaking.
Discover How NRI Graduate Student Fights Fetal Alcohol Spectrum Disorder
Hannah Petry, a third-year doctoral student at the UNC Nutrition Research Institute, was recently awarded one of only eight prestigious travel awards. This award granted her the opportunity to present her groundbreaking research at the Fetal Alcohol Spectrum Disorders...
Virtual Internship Program is accepting applications
Impact Report FY23
Molding the Scientific Minds of Tomorrow
The UNC Nutrition Research Institute (NRI) is helping mold the scientific minds of tomorrow by offering unique learning and work experiences for graduate students in sciences related to personalized nutrition, like nutrigenomics and metabolomics. These young...
2023
2020
2019
2017
2016